
Guest post by David Vailencourt, M.Sc., Founder and CEO, The GMP Collective.
Surna and The GMP Collective work with folks all over the world who must adhere to different rules and regulations for cannabis cultivation, storage, and manufacturing. Historically, we saw emphasis on Good Manufacturing Practices (GMP) compliance from our international clients, notably Europe and Canada. However, as new states legalize cannabis and existing states continue to tweak their regulations in an effort to harmonize the industry, a significant uptick in inquiries about GMPs has occurred right here in our backyards. And for good reason.
Many people understand GMP compliance to relate specifically to cleanliness standards, and while that is absolutely part of the equation, it also relates to the actual processes that are undertaken to ensure repeatable manufacturing processes. With heating, ventilation, and air conditioning (HVAC) design, that means proper, consistent and tightly controlled parameters, effective filtration, consistent maintenance procedures, and airflow patterns specifically designed for avoidance of cross contamination, among other things. In this article, we will analyze several major considerations:
- What is GMP?
- Getting Started
- HVAC and Facility Design
- Regulatory Requirements
What Are GMPs for Indoor Cannabis Cultivation?
GMP refers to a system of manufacturing that guarantees reproducibility of product quality to set specifications. Federally, GMPs are required for the dietary, food, and pharmaceutical industries and can be found in Chapter 21 of the Code of Federal Regulations (notably Parts 111, 117, and 211 respectively), as well as globally as established by the World Health Organization (WHO) and standards development organizations.
The details can be intimidating, especially to a newcomer. However, diving deeper into the meaning behind them unveils the benefits that organizations realize upon successful implementation. GMPs bring credibility to a business (and an industry), mitigate risks, and ensure final products are safe and consistent. No other system in the world has been developed and adopted that provides this level of much-needed safety.
GxP, GACP, GMP: What Does it Mean?
First, let’s make this alphabet soup more digestible. If “x” represents a variable, then “GxP” refers to a general set of Good Practices within a variable application. They can be tailored to manufacturing (GMP), agricultural and collection (GACP), distribution (GDP), laboratory (GLP) and more. For cultivators, GACP is your soup du jour. If you are a processor or manufacturer, GMP is your language. Regardless, the principles are the same, so read on!
Where to Begin – What’s Your Goal?
The GMP term for this is User Requirement Specifications (URS). Some may call them owners’ Key Performance Indicators (KPIs). Whatever you call them, it’s critical to start with the end in mind and is as simple as brainstorming to answer some basic questions.
- What product line(s) do you intend to produce and store?
- What are your major processing steps (i.e., bulk formulations, filling/packaging and testing, product ingredient and component specifications and storage conditions)?
- What raw materials, ingredients, personnel, and systems will be needed to carry out each one of these activities?
- What checks need to be in place to verify that the process resulted in the desired end product?
- What capacity do you need to meet (output and throughput), and how does this relate to inventory and quantities to be on order and in stock?
- How will materials flow through the facility as part of your identified processes? Think process flow maps – this will help identify the need for pass-thrus, interlocks, and establishing different work zones.

By answering these questions, you’ll begin to understand what your processing constraints, building conditions, etc. are. These processing constraints, or bottlenecks, are an operations pain point. They limit your throughput, can lead to storage issues, and are generally inefficient. Translation – it becomes more expensive to operate per gram of product than your competitor who thought this through!
HVAC, Facility Design, and GxP
GxPs at a fundamental level ensure that you are able to meet your end goal (producing safe, high-quality products at a profit) through identifying the risks inherent in the process and applying control measures to prevent brand-damaging product failures and recalls.
First, you need to characterize the riThink biological (pathogens like mold and mildew), chemical (pesticides, heavy metals, and other impurities), and physical (dirt, dust, metal fragments, glass) that are caused from the movement of people and materials from one area to another. This informs your HVAC needs since air exchanges and filters within your HVAC system help mitigate pathogens, dust, and more. You should also consider what cleaning and sanitation procedures you will implement and at what frequency will you need to perform them. Again, these are answers that will surface from this initial exercise.
This exercise will provide you with the lens and criteria in which you can evaluate your overall design. Humans tend toward the path of least resistance, so if your facility is designed inefficiently for your workflow, your team will likely come up with ways to shortcut the long hallways and pass through rooms that inadvertently become vectors for contaminating your operation.
A team of experienced engineers, architects, and cannabis and GMP experts are your best insurance policy. For instance, Surna has seen the good, bad and the ugly of decisions made during the design and construction process and will help you mitigate those risks through efficient design controls. Think GMP is expensive? Try a poorly designed HVAC system and grow facility layout that does not have GMP principles built in! Systemic pathogen outbreaks, seasonal challenges, and more will plague your operation until the end of time.
GMP programs build off of a smart design and enable your operation to produce high quality and safe products long after the commissioning agents have left. Better design = less GMP controls = less operational problems = success. Ultimately, a smart and efficient design will translate to considerable operational savings.
Futureproof Your Business – Regulatory Requirements
The writing is on the wall, and it is important not to wait until regulators mandate best practices to ensure your facility is clean, safe, and able to produce consistent passing products. Look at the industry trends of adopting GMPs in many marketplaces, and look deeper to the history of the food, spice, agricultural, and medical industries.
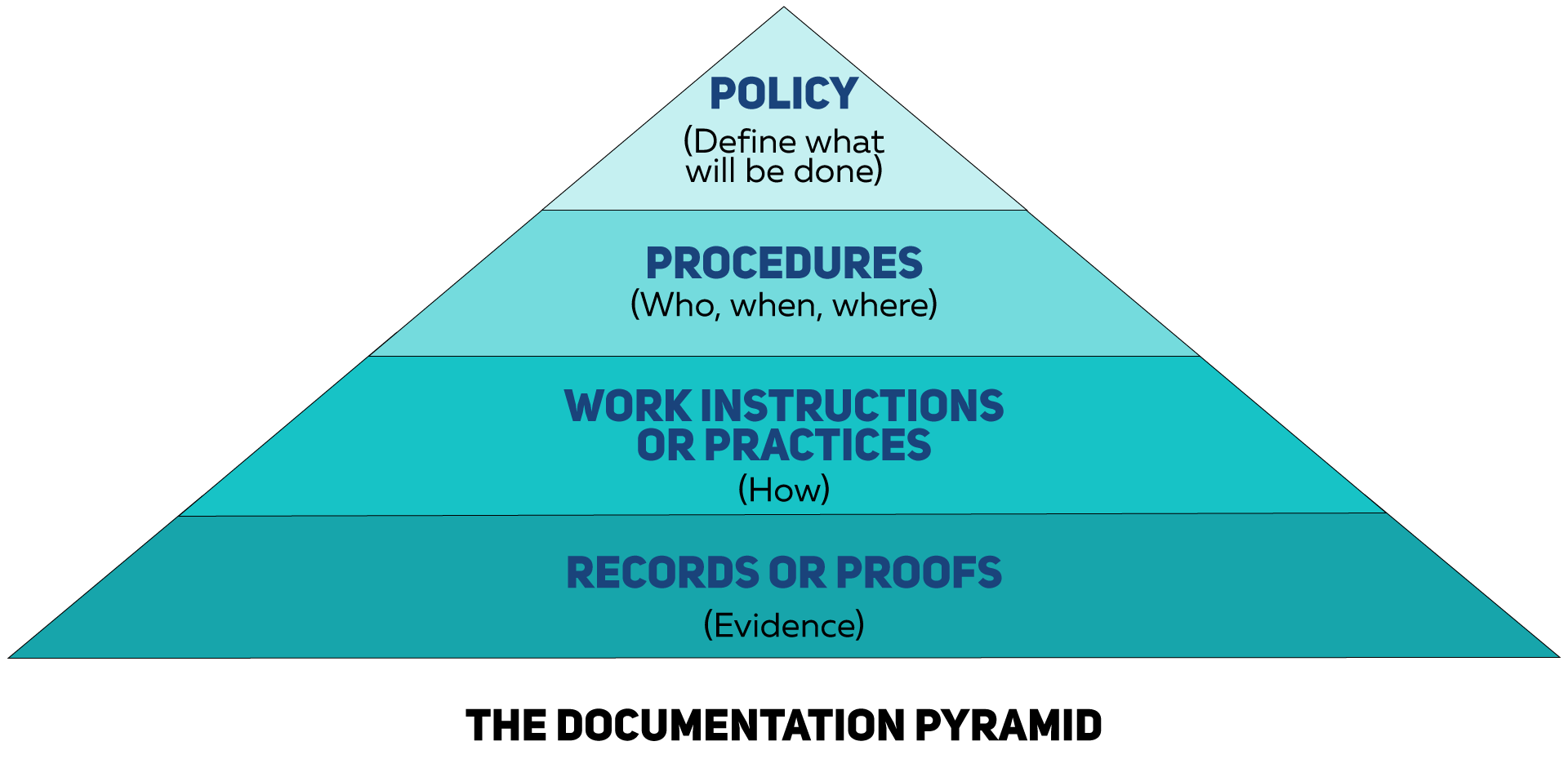
What Do I Need to Be Able to Explain and Document?
I’ve had some people refer to GMPs as “Get More Paper,” and in some respects, they are not wrong. Paperwork is tangible evidence that something actually occurred. After the walls are closed up, the filter cover is secured, and employees have gone on vacation or moved on to other roles, documentation is the only evidence you have to confirm what you stated you planned to do was done. The figure below shows examples of key procedures, their purposes, and what types of measurements (test parameters) one would use to verify.
| Test Procedure and Key Aspects | Considerations | Objective | Test Parameter |
| Particle counter readings & positions | Number of readings and positions | Verifies cleanliness | Particle count test |
| Measure pressure difference | Continuous. Defined limits | Absence of cross-contamination | Air pressure difference |
| Measure supply and return air, calculate air change rate | See ISO 14644 | Verify air change rates | Airflow volume |
| Velocity measurement | Velocity and containment | Verify unidirectional airflow and/or containment condition | Airflow velocity |
| Temperature and humidity | Product specifications affected (hot or cold, moisture content) Equipment & personnel load in room | Maintain optimal product protection and personnel comfort | Electronic (constant) or manual temperature/RH reading and recording |
Key Take-Aways
Many companies take their HVAC systems and overall designs for granted. Do not simply assume that your system will deliver proper temperatures, air flow, room pressure differentials and humidity control without considering the capabilities of the existing system or its limitations, condition or age.
In order to depend on reliable performance of your HVAC and facility, elicit a professional engineering company. With the stated specifications of the client, they can design a proper system that meets GMP requirements and significantly reduces overall operational costs.
GMPs are not only becoming increasingly required of the cannabis industry, but are also a timeless and proven tool that allows businesses to position themselves for long term success. For more resources on GMPs in the cannabis industry, start here:
- “Committee Blog: Facts About Current Good Manufacturing Practices (cGMPs) And Their Role In The Cannabis Industry”, https://thecannabisindustry.org/committee-blog-facts-about-current-good-manufacturing-practices-cgmps-and-their-role-in-the-cannabis-industry/
- “GMPs start at your property line”, https://www.gmpcollective.com/post/gmps-start-at-your-property-line
- “GMPs and cannabis manufacturing” https://cannabisindustryjournal.com/feature_article/gmps-cannabis-manufacturing/
- “Why GMP Certification matters for cannabis industry” https://www.naturalproductsinsider.com/regulatory/why-gmp-certification-matters-cannabis-industry
Who is The GMP Collective?
The GMP Collective is a hands-on consulting firm that is dedicated to ensuring the quality, compliance, and safety of cannabis and cannabinoid containing products. They lead discussions with regulators, industry, and consumers globally on harmonization efforts that are bringing credibility and protecting our industry daily. Their team is comprised of quality, operational, and compliance experts with decades of experience in the life sciences and consumer packaged goods (CPGs) industries. The Collective effectively and passionately applies globally established practices and standards required by producers of drugs, supplements, and food to the emerging cannabis products space.

